Clinical Trial Safety: What You Need to Know About Drug Testing Risks and Protections
When a new drug enters the market, it doesn’t just appear out of nowhere—it goes through clinical trial safety, a structured process to test whether a medication is safe and effective in humans before it’s approved for public use. This isn’t just paperwork; it’s a system designed to protect real people from harm while finding treatments that work. Without these safeguards, drugs could cause serious side effects, even death. That’s why every phase of a trial—whether it’s testing on 20 volunteers or 10,000 patients—is tightly monitored.
FDA regulations, the rules enforced by the U.S. Food and Drug Administration to oversee drug development and approval set the baseline for what’s allowed in trials. These rules require researchers to report every side effect, no matter how small, and stop a trial if risks outweigh benefits. Adverse events, unintended and harmful reactions to a drug during testing are tracked in real time. If a participant gets sick, the team must document it, analyze it, and decide whether to keep going. This isn’t theoretical—it’s how drugs like statins, diabetes meds, and even cancer treatments were proven safe enough for millions to use.
Participants aren’t left on their own. Before joining a trial, you’re given clear info about what to expect, including possible risks. Informed consent isn’t just a signature—it’s a conversation. Trials also have independent review boards that watch for red flags, and many include data safety monitoring committees that can halt a study without warning if something goes wrong. Even after a drug is approved, safety doesn’t end. Post-market surveillance keeps watching for rare side effects that only show up in large populations.
What you’ll find in the articles below are real-world examples of how safety works—or fails—in practice. From how barcode scanning in pharmacies prevents errors to why grapefruit juice can interfere with meds, these posts show how small details matter in the bigger picture of drug safety. You’ll see how deprescribing reduces risks in older adults, why black box warnings exist, and how automated refills help people stay on track without missing doses. These aren’t random topics—they’re all connected to the same goal: making sure medications do more good than harm.
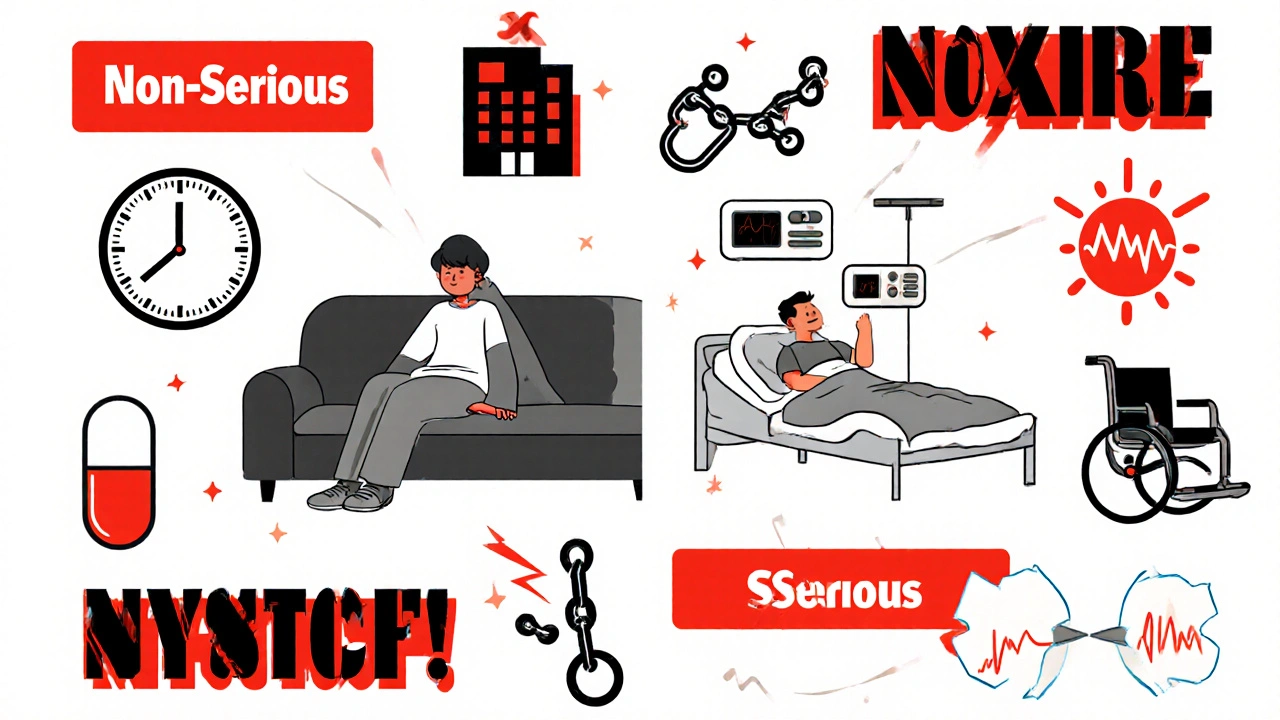
Serious vs Non-Serious Adverse Events: When to Report in Clinical Trials
Learn the critical difference between serious and non-serious adverse events in clinical trials. Understand when to report each type, why the distinction matters for patient safety, and how to avoid common reporting mistakes.