Why Bioequivalence Studies Used to Ignore Women and Older Adults
For decades, bioequivalence studies-the clinical tests that prove a generic drug works just like the brand-name version-were done almost exclusively on young, healthy men. It wasn’t because scientists thought men were better test subjects. It was easier. Men were more available, didn’t face pregnancy risks, and had less hormonal variation. But this approach ignored a simple truth: people don’t all absorb drugs the same way.
Women, older adults, and even people with different body types process medications differently. A drug that works perfectly in a 25-year-old man might be too strong or too weak in a 70-year-old woman with slower kidney function. Yet, for years, regulators accepted data from these narrow studies to approve generics for everyone. That’s changing.
What Bioequivalence Actually Means
Bioequivalence isn’t about whether two pills look the same. It’s about whether they deliver the same amount of active drug into your bloodstream at the same speed. If a generic version of your blood pressure pill doesn’t match the brand in absorption, you could get too little (and risk a stroke) or too much (and risk dangerous drops in blood pressure).
Regulators like the FDA and EMA use blood tests to measure two things: how much of the drug gets into your system (AUC) and how fast it gets there (Cmax). If the generic’s values fall within 80-125% of the brand’s, it’s considered bioequivalent. Simple, right? Not quite. These numbers can shift based on age, sex, and even body weight. And if the study group doesn’t reflect real users, the results might be misleading.
Age Matters More Than You Think
As we age, our bodies change. Liver enzymes slow down. Kidneys filter less efficiently. Fat and muscle ratios shift. All of this affects how drugs are absorbed, distributed, and cleared.
The FDA now requires that if a drug is meant for older adults-like those used for Alzheimer’s, arthritis, or heart failure-bioequivalence studies must include participants aged 60 and older. If they don’t, the sponsor must explain why. The EMA doesn’t mandate it, but expects justification if elderly people are left out. ANVISA in Brazil draws the line at 50 years old.
Here’s a real example: a generic version of a common antidepressant was tested in healthy 20-year-olds and passed bioequivalence. But when tested in adults over 65, the older group had 30% higher drug levels in their blood. That’s not a small difference-it could mean more dizziness, falls, or confusion. The drug stayed on the market, but the manufacturer had to add a warning for older patients.
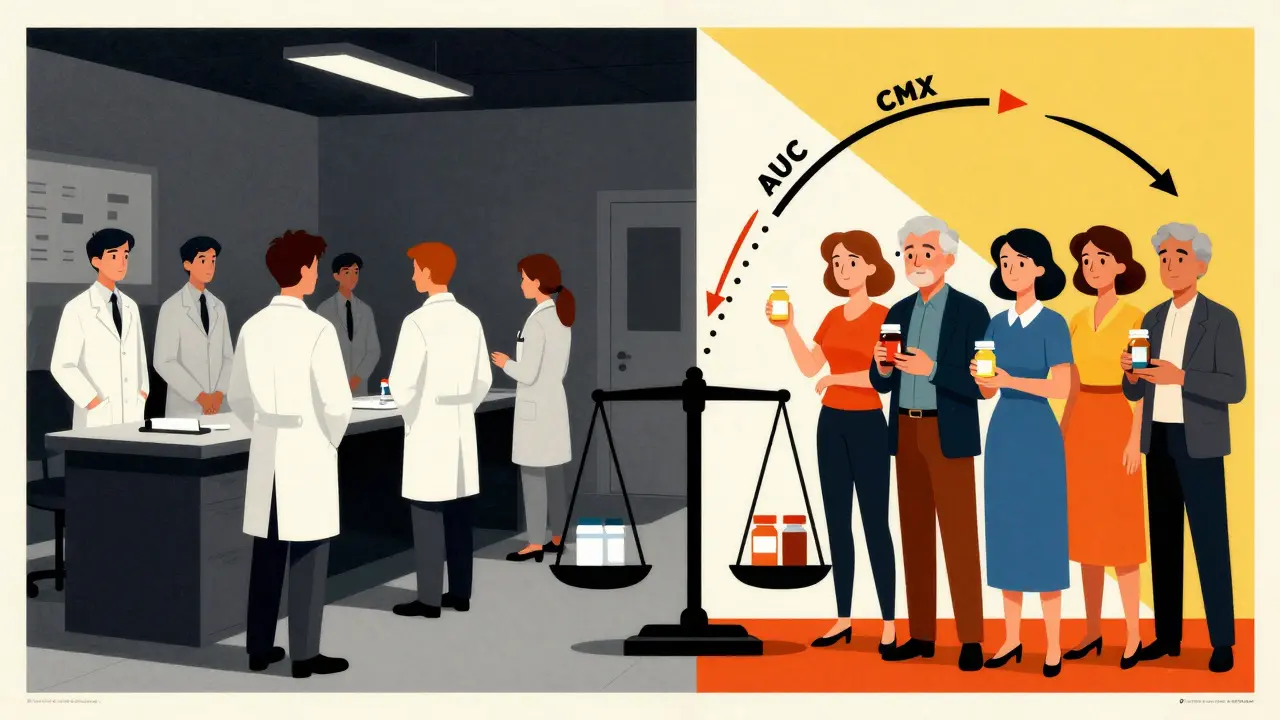
Sex Differences Aren’t Just About Hormones
It’s not just about estrogen and testosterone. Women tend to have lower body weight, higher body fat, slower gastric emptying, and different enzyme activity than men. These differences affect how quickly a drug enters the bloodstream and how long it lasts.
Take levothyroxine, the most prescribed thyroid medication. Over 60% of users are women. Yet, in many bioequivalence studies, fewer than 25% of participants were female. That’s a problem. In one 2021 study, women showed 18% higher peak concentrations than men after taking the same generic pill. The difference didn’t break bioequivalence rules-but it could mean women need lower doses to avoid side effects like anxiety or heart palpitations.
The FDA’s 2023 draft guidance now says: if a drug is used by both sexes, the study must include roughly equal numbers of men and women. No more excuses. The EMA still says subjects “could belong to either sex,” which sounds open-ended but often means companies pick the easiest group: men.
How Regulations Differ Around the World
Regulatory rules aren’t the same everywhere. Here’s how the major agencies compare:
| Agency | Minimum Age | Maximum Age | Sex Balance Required? | Healthy Volunteers Only? |
|---|---|---|---|---|
| FDA (USA) | 18 | None (60+ encouraged) | Yes, ~50:50 unless justified | No (chronic conditions allowed) |
| EMA (Europe) | 18 | None | No, but must be representative | Yes |
| ANVISA (Brazil) | 18 | 50 | Yes, equal distribution | Yes |
| Health Canada | 18 | 55 | Recommended, not required | Yes |
The FDA is the most flexible on health status-allowing people with stable chronic conditions like controlled hypertension to participate. That’s smart. If you’re taking a drug for high blood pressure, you’re not a “healthy volunteer.” You’re the actual patient. The EMA and ANVISA still stick to strict healthy volunteer rules, which limits how well the results reflect real-world use.
Why Gender-Balanced Studies Are Hard to Run
It sounds simple: recruit half men, half women. But it’s not.
Women are less likely to join clinical trials. They juggle caregiving, have more scheduling conflicts, and worry about pregnancy risks-even if they’re using contraception. Sites report recruitment takes 40% longer for gender-balanced studies. And it costs more. One CRO estimated a 25% increase in budget just to reach equal representation.
Then there’s the statistical problem. Small studies (n=12-14) can give false signals. In one case, a generic drug looked like it worked differently in men versus women. But when the study was repeated with 36 participants, the difference vanished. That’s because tiny studies are easily skewed by one or two outliers. Bigger groups smooth out the noise.
That’s why experts now recommend stratified randomization-making sure men and women are evenly split across the drug sequences-and pre-planned subgroup analysis. You don’t just check if the drug is bioequivalent overall. You check if it’s bioequivalent in men and in women separately.
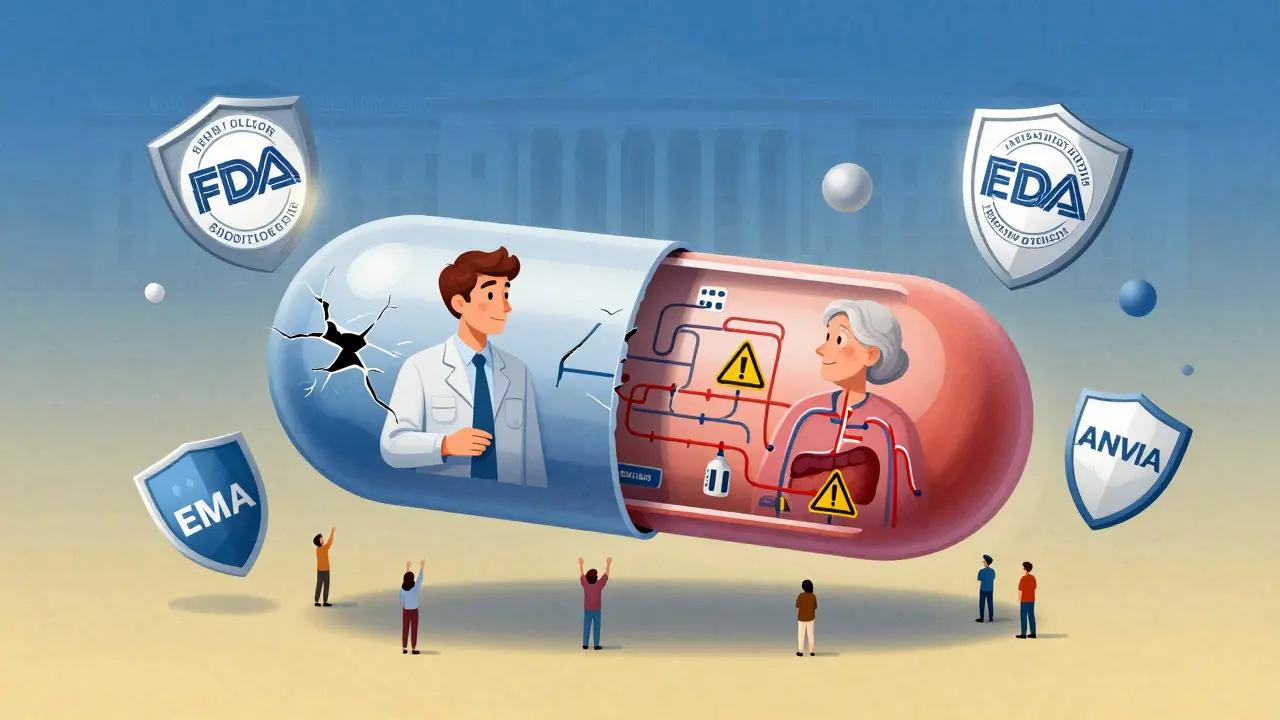
The Real-World Cost of Ignoring Diversity
Ignoring age and sex isn’t just bad science-it’s dangerous.
Studies show that women experience adverse drug reactions 50-75% more often than men. Why? Because most drug dosing was based on male physiology. A 2023 University of Toronto study found that 37% of commonly tested drugs are cleared 15-22% faster in men. That means women might need higher doses to get the same effect. Or, if they’re given the same dose, they might build up toxic levels over time.
Take warfarin, a blood thinner. Women over 60 often need lower doses than men. But if a generic version was tested only on young men, the dosing instructions might not reflect that. Patients end up on the wrong dose. Hospitals see more bleeding events. Pharmacies get more calls.
And it’s not just women. Older adults are often excluded, even when they’re the main users of the drug. A 2020 FDA review found that 70% of drugs approved for seniors had bioequivalence data from participants under 50.
What’s Changing-and What’s Still Lagging
The tide is turning. The FDA’s 2023 draft guidance is the strongest signal yet: balance isn’t optional. If you’re making a drug for both men and women, you need both in your study. The EMA is reviewing its 2010 guidelines and may follow suit. ANVISA already requires equal sex distribution.
But adoption is slow. In a review of 1,200 generic drug applications from 2015 to 2020, only 38% had female participation between 40-60%. The median was 32%. That’s not representation. That’s tokenism.
Companies are catching on. Sixty-eight percent of contract research organizations now actively recruit women. Some offer childcare, flexible hours, and transportation. But only 29% track sex-specific pharmacokinetics in their reports. That’s the next hurdle: not just including women, but analyzing how the drug behaves in them.
What This Means for Patients
You don’t need to understand AUC or Cmax. But you should know this: your generic pill was tested on people who might not look like you. That’s changing. Regulators are finally asking: Who are we really testing for?
If you’re a woman over 50 taking a daily pill, you deserve to know that the generic version was tested on people like you-not just on 22-year-old men. And now, thanks to updated rules, that’s becoming the norm.
The goal isn’t to make studies harder. It’s to make them smarter. To make sure that when you pick up your prescription, you’re getting a drug that works for your body-not someone else’s.
Why were bioequivalence studies historically done only on young men?
Early bioequivalence studies focused on young, healthy men because they were easier to recruit, had fewer variables like hormonal cycles or pregnancy risks, and were seen as the most consistent group for measuring drug absorption. This practice persisted despite known differences in how men and women metabolize drugs, largely due to convenience and outdated assumptions about physiological uniformity.
Does sex really affect how a generic drug works?
Yes. Women often have slower gastric emptying, higher body fat, lower liver enzyme activity, and different kidney filtration rates than men. These factors can lead to higher drug concentrations in women, especially for medications cleared by the liver. Studies have shown differences in peak drug levels and duration between sexes for drugs like antidepressants, blood thinners, and thyroid medications-even when the overall bioequivalence criteria are met.
Are older adults included in bioequivalence studies?
It depends on the regulator and the drug. The FDA requires inclusion of adults aged 60+ for medications intended for seniors, unless there’s a strong scientific reason not to. The EMA and ANVISA don’t mandate it, but expect justification for excluding older adults. Many studies still skip them, even though older people are often the main users of these drugs.
What’s the minimum number of participants needed in a bioequivalence study?
The EMA requires at least 12 evaluable subjects, but most studies enroll 24-36 to account for dropouts and ensure enough power to detect differences, especially when analyzing subgroups like sex or age. Small studies (under 20 participants) can produce misleading results due to outliers, so larger samples are now standard.
Can a bioequivalence study done on men be used to approve a drug for women?
Regulators used to allow it-but no longer. The FDA’s 2023 draft guidance explicitly says that if a drug is intended for both sexes, the study must include similar proportions of males and females. The EMA still allows it with justification, but experts warn this practice risks underdosing or overdosing women, leading to adverse effects.
How can patients know if a generic drug was tested on people like them?
Currently, this information isn’t always public. But as regulators require more transparent reporting, sponsors must include demographic data in clinical study reports. Patients can ask their pharmacist or doctor if the generic was tested in populations similar to theirs-especially if they’re over 60, female, or have chronic conditions. In the future, this data may be included in drug labeling or public databases.




15 Comments
kate jones- 1 February 2026
This is long overdue. For years, we've been treating pharmacokinetics as if everyone's a 25-year-old male with perfect liver function. The data doesn't lie-women and older adults metabolize drugs differently, and ignoring that isn't science, it's negligence. The FDA's 2023 guidance is a step in the right direction, but enforcement needs teeth. We need mandatory subgroup analysis in all applications, not just "encouraged" inclusion.
Natasha Plebani- 2 February 2026
It's fascinating how we built an entire regulatory framework on the assumption of physiological homogeneity, then wonder why adverse events are higher in marginalized populations. The real issue isn't just recruitment-it's epistemological. We've been measuring bioequivalence through a male, young, able-bodied lens and calling it universal. That's not science. That's bias dressed in lab coats.
owori patrick- 3 February 2026
In Nigeria, we don't even have the luxury of these debates. Many generics are imported without any demographic data at all. If a pill works for a 30-year-old man in Boston, we assume it works for a 68-year-old woman in Lagos. The cost? Higher hospitalizations, more deaths. This isn't just an American problem-it's a global failure of equity in medicine.
April Allen- 4 February 2026
The 80-125% window is a statistical abstraction that masks real biological variation. Two drugs can be "bioequivalent" on paper but behave completely differently in a 70-year-old woman with renal impairment. We need pharmacokinetic modeling that accounts for covariates-age, sex, BMI, comorbidities-not just a single cohort of healthy volunteers. The science is there. The will isn't.
Kathleen Riley- 4 February 2026
It is imperative to acknowledge that the historical exclusion of non-male, non-young participants from bioequivalence trials constitutes a systemic epistemic injustice within the pharmaceutical regulatory paradigm. The ontological privileging of the young male body as the default human model has led to a profound misalignment between clinical evidence and population-level therapeutic outcomes.
Beth Cooper- 5 February 2026
Let’s be real-this isn’t about science. It’s about control. Big Pharma doesn’t want to test on women and elderly because then they’d have to make different dosages. And guess what? That means more pills. More profits. They’d rather keep people sick than lose revenue. They’ve been hiding behind "bioequivalence" to avoid having to reformulate. Wake up.
Donna Fleetwood- 5 February 2026
I love that this is finally getting attention. I’m 58 and on three meds-none of which were tested on people my age. It’s scary. But change is happening, slowly. I’ve started asking my pharmacist: "Was this tested on someone like me?" And you know what? They’re starting to look it up. Small steps, but they matter.
Melissa Cogswell- 6 February 2026
The biggest hurdle isn't recruitment-it's analysis. Many studies include women but don't report sex-stratified PK data. Without that, you can't tell if the drug behaves differently. I've reviewed dozens of submissions where "n=30" included 15 women... but the results were only reported as pooled. That's not inclusion. That's window dressing.
Shubham Dixit- 7 February 2026
In India, we export 40% of the world’s generics. But we still follow EMA guidelines that allow male-only studies. Why? Because it’s cheaper. But when a woman in Germany has a stroke because her generic blood thinner was tested only on Indian men, who is responsible? We need global standards. Not just American ones. This isn't a Western problem-it's a global accountability crisis.
Blair Kelly- 9 February 2026
I’ve seen this firsthand. A generic version of a cardiac drug I was on was approved based on a study with 14 healthy 22-year-old men. I’m a 54-year-old woman. I had palpitations for weeks. Turns out, my blood levels were 40% higher. The FDA approved it. The manufacturer didn’t even update the label. This isn’t oversight. It’s corporate malpractice.
Rohit Kumar-10 February 2026
The real tragedy is that we already know the science. Pharmacokinetic differences by sex and age have been documented since the 1980s. What’s lacking is political will. Regulatory agencies are too cozy with industry. They accept "justification" as a loophole. We need mandatory inclusion thresholds-not suggestions. And we need independent audits. Not industry-funded ones.
Kimberly Reker-11 February 2026
My mom’s on a generic antidepressant. She started having dizzy spells. We looked into it-turns out the study was done on college students. She’s 72. No one told us. This isn’t just about data-it’s about trust. We need transparency. Like, publicly accessible demographic reports for every generic approval. No more "confidential sponsor data".
Rob Webber-11 February 2026
I work in pharma. We’re told to "aim for 50/50" but then pressured to cut costs. So we recruit 20 men and 10 women, call it "representative," and move on. The statistical power is garbage. We’re not fixing the system-we’re gaming it. And patients are paying with their health. This isn’t innovation. It’s exploitation.
calanha nevin-11 February 2026
The regulatory landscape must evolve to reflect the diversity of the patient population. Exclusionary trial design perpetuates health disparities. The inclusion of older adults and women is not a social initiative-it is a pharmacological imperative. Failure to stratify analysis by sex and age constitutes a violation of the principle of beneficence in clinical research.
Lisa McCluskey-12 February 2026
I’m glad this is getting attention. But let’s not pretend it’s just about sex and age. What about race? Genetics? Gut microbiome? We’re still treating bioequivalence like a one-size-fits-all equation. The next frontier is personalized PK modeling. Until then, we’re just patching holes in a sinking ship.