Tag: generic drugs
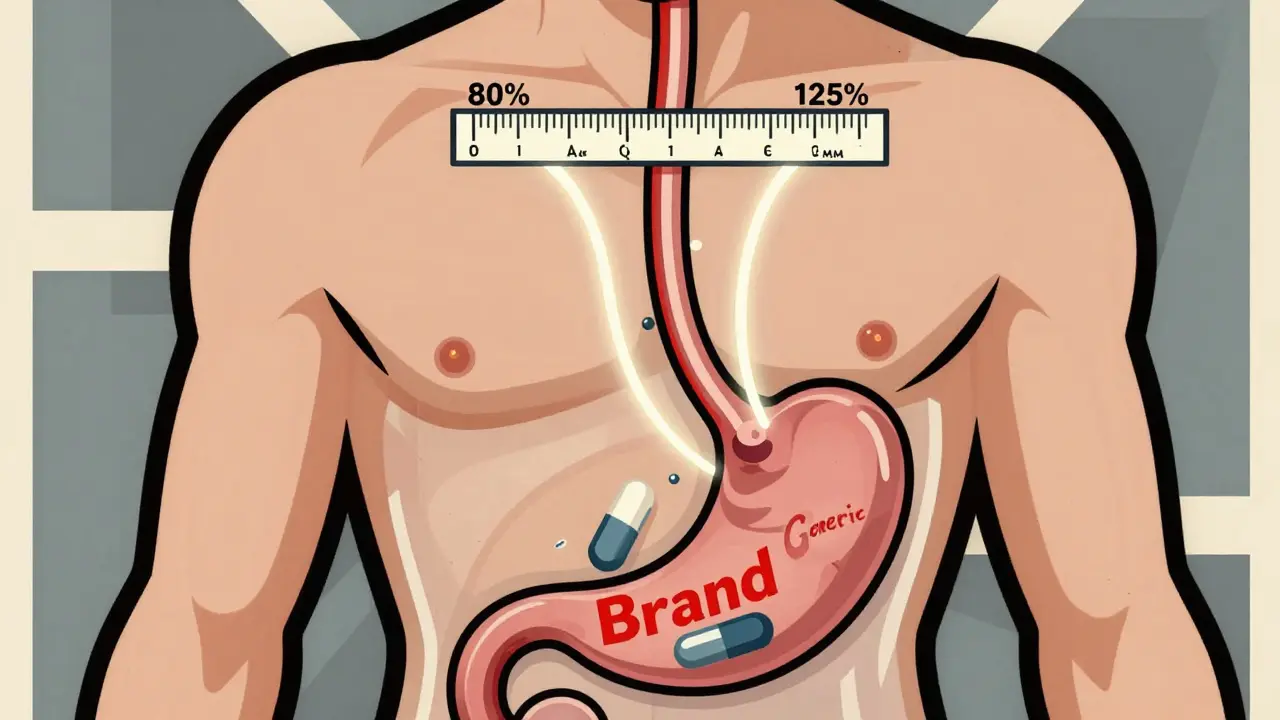
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals for Generic Drugs
The 80-125% rule ensures generic drugs are absorbed the same way as brand-name versions. It's not about drug content-it's about how your body processes it. Here's how it works, why it matters, and what it really means for patients.
Consumer Language Guides: Making Generic Drug Information Accessible
Consumer language guides help patients understand that generic drugs are just as effective as brand-name ones-same active ingredient, same results, much lower cost. Learn how plain-language tools are breaking down myths and boosting adherence.
Special Populations in Bioequivalence: How Age and Sex Impact Generic Drug Testing
Bioequivalence studies for generic drugs have historically excluded women and older adults. New regulations now require more representative testing to ensure safety and effectiveness across all users.
Drug Interactions: Same Risk for Generic and Brand Medications
Generic and brand-name drugs have the same active ingredients and carry the same risk of drug interactions. The FDA and major studies confirm they're equally safe and effective. Here's what you really need to know.
Prescriber Attitudes Toward NTI Drugs and Substitution: What Doctors Really Think
Prescribers remain cautious about substituting generic drugs for narrow therapeutic index medications due to risks of instability and adverse effects. Despite FDA assurances, doctors prioritize patient safety through careful monitoring and controlled switching.
How Generic Drugs Save Billions in the U.S. Healthcare System
Generic drugs save the U.S. healthcare system nearly $500 billion annually by providing the same effectiveness as brand-name medications at a fraction of the cost. Discover how generics and biosimilars are reshaping drug pricing and what you can do to save money.
How Media Coverage Undermines Confidence in Generic Drugs
Despite being just as safe and effective as brand-name drugs, generics face widespread public mistrust fueled by misleading media coverage. Learn how news stories shape perceptions - and what can be done to restore confidence.
Hatch-Waxman Amendments: How Landmark Law Created the Modern Generic Drug Market
The Hatch-Waxman Act of 1984 created the modern system for generic drugs in the U.S., balancing innovation incentives with affordable access. It cut approval costs by 90%, boosted generic use to 90% of prescriptions, and sparked ongoing debates over drug pricing.
Why Generic Medications Cost Less for Patients and Insurers
Generic medications cost far less than brand-name drugs because they don't need to recoup R&D costs or run expensive marketing campaigns. They're just as safe and effective, and they save patients and insurers hundreds of billions each year.