Category: Medications - Page 2
Consumer Language Guides: Making Generic Drug Information Accessible
Consumer language guides help patients understand that generic drugs are just as effective as brand-name ones-same active ingredient, same results, much lower cost. Learn how plain-language tools are breaking down myths and boosting adherence.
Hypothyroidism and Statins: How Uncontrolled Thyroid Levels Raise Myopathy Risk
Hypothyroidism increases the risk of statin-induced muscle damage. Learn how uncontrolled thyroid levels raise myopathy risk, which statins are safest, and what steps to take to protect your muscles and heart.
Special Populations in Bioequivalence: How Age and Sex Impact Generic Drug Testing
Bioequivalence studies for generic drugs have historically excluded women and older adults. New regulations now require more representative testing to ensure safety and effectiveness across all users.
Reimbursement and Coding for Biosimilars: How Billing Works Under Medicare Part B
Biosimilars have unique billing rules under Medicare Part B. Learn how HCPCS codes, ASP-based reimbursement, and the JZ modifier impact provider payments and adoption rates.
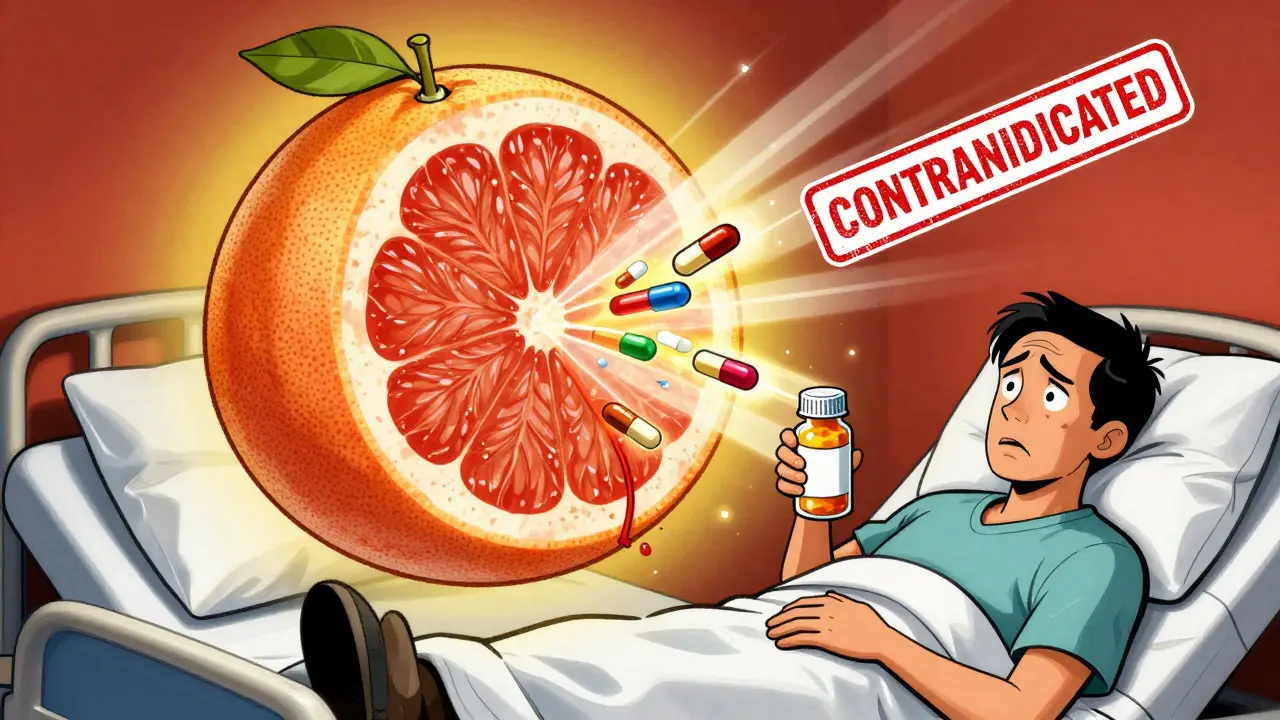
Grapefruit and Immunosuppressants: What You Need to Know Before You Eat It
Grapefruit can dangerously raise levels of immunosuppressants like cyclosporine and tacrolimus, leading to kidney damage or organ rejection. Even small amounts can cause toxic effects that last up to 72 hours. Avoid grapefruit entirely if you're on these medications.
Drug Interactions: Same Risk for Generic and Brand Medications
Generic and brand-name drugs have the same active ingredients and carry the same risk of drug interactions. The FDA and major studies confirm they're equally safe and effective. Here's what you really need to know.
Adverse Event Reporting: What Pharmacists Must Know About Generic Medication Safety
Pharmacists play a critical role in detecting and reporting adverse events from generic medications. Learn why their reports matter, how to report correctly, and how under-reporting puts patients at risk.
Migraine Medications: Triptan Interactions and Limitations
Triptans are effective for migraines but come with serious interactions and limitations. Learn when they work, when they fail, and which drugs to avoid mixing with them.
Antiviral Medications: Treatment Options for Viral Infections
Antiviral medications like Paxlovid, Tamiflu, and hepatitis C cures help treat viral infections by stopping viral replication. Learn how they work, which ones are most effective, and why timing and access matter.
Tyramine-Rich Foods and MAO Inhibitors: What You Must Avoid to Prevent Hypertensive Crisis
MAO inhibitors can save lives for treatment-resistant depression-but eating the wrong foods can cause a dangerous blood pressure spike. Learn which foods to avoid, how newer MAOIs reduce risks, and what to do if you accidentally eat tyramine.